Why does water expand when it freezes?
On heating, liquids expand since the molecules move with greater energy overcoming the intermolecular attraction. On the contrary, liquids usually contract on cooling. But that is not the case with water.
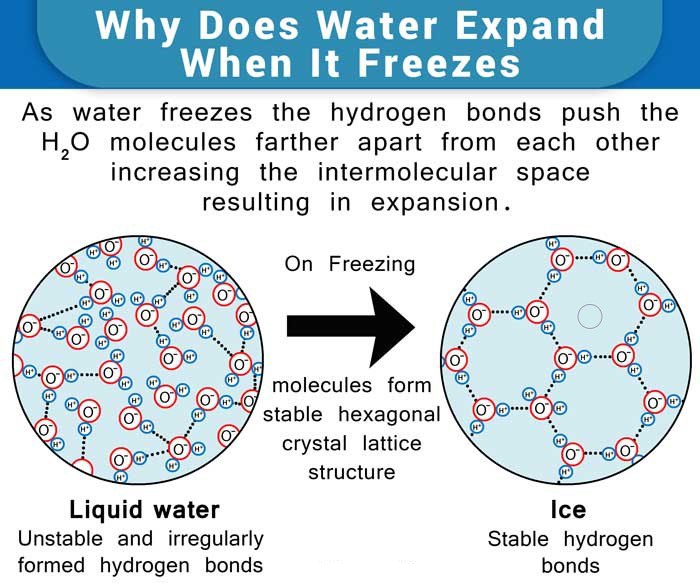
The water molecules consist of 2 atoms of hydrogen and one of oxygen, the oxygen atom side being slightly negative, while the hydrogen atoms side – slightly positive, forming hydrogen bonds. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. Hence, water expands upon freezing and becomes less dense. On the other hand, it contracts on thawing, much unlike most other liquids.
Water expands approximately by about 10%.
Water is not the only substance that expands when it freezes, other substances are plutonium, germanium, bismuth, gallium, silicon, etc.
This phenomenon,
the anomalous expansion of water, also explains why ice is floating in water.
Subscribe- t.me/askmenow